Describe Solids Liquids and Gases Using Scientific Terms
Solid vibrate jiggle but generally do not move from place to place. When at normal temperatures between 0 o C and 100 o C it is a liquid.

Properties Of Solids Liquids Gases Compared Teachoo Science
The particles in solids are very close.

. Liquids and solids are often referred to as condensed phases because the particles are very close together. Gas refers to a state of matter do not have any shape but conform to the shape of the container completely in which it is put in. Gases have neither a fixed volume nor a fixed shape.
When solid the molecules in water are held tightly together and dont move easily. What determines the phase of a substance. A liquid has a defined volume but can change state.
The process is called condensation. Solids liquids and gases The kinetic particle theory of matter is a model that describes the arrangement movement and energy of particles in a substance. The familiar compound H 2 O provides the evidence that substances occur in three different physical classes called states of matter.
The state that water is in depends upon the temperature. Now up your study game with Learn mode. Terms in this set 46 Matter.
Gas water is called steam or vapor. Difference between Solid Liquid and Gases. The particles in solids liquids and gases have _____ amounts of energy.
At low temperatures below 0 o C it is a solid. There are lots of. Solids Liquids and Gases.
Solids Liquids and Gases. Whether or not they hold their volume and shape. These are what we call the three states of matter.
Start studying Science Vocab. Solid refers to a form of matter which has structural rigidity and has a firm shape which cannot be changed easily. Solids Liquids and Gases Chapter 2.
Lots of materials are solid such as paper bricks wood metal and ice. The particles in liquids. Solid carbon dioxide is called dry ice because it converts from a solid to a gas directly without going through the liquid phase in a process called sublimation.
Unit 4 Study Guide 5th grade Science Bear Lake Elementary Apopka Florida. The substance we are probably most familiar with has those three phases. A solid at a particular temperature has a fixed shape and volume which may be affected by changes in temperature.
The solid the liquid and the gas phase. The amount of energy in molecules of matter determines the state of matter. Solid water is called ice.
Plasma is the fourth state of matter. If the liquid is heated to 100C however it abruptly expands to a tenuous fluid called vapor or gas. Has mass and volume.
The kinetic energy of particles is higher than in solids and liquids. While at temperatures above 100 o C water is a gas steam. Water can take many forms.
A gas is a state of matter in which atoms or molecules have enough energy to move freely. 2 terms used to describe a liquid into a gas. Solids usually increase slightly in size when.
Move close together. Solids liquids and gases Solids. An example of gases.
It is important to know the major differences between solids liquids and gases. Liquid water is just called water. A gas turns into a liquid state and it is condensed.
A common example is ice. Generally the strength of the intermolecular interactions determines whether a substance is a solid liquid or gas under any particular conditions. A solid changes into a liquid under the process of melting.
As ice heats up it will change phases to liquid water. It describes the arrangement movement and energy of particles in a. An example is liquid water.
At room temperature H 2 O is a dense fluid called a liquid. Several exotic states also exist. Covalent network bonding is a very strong form of intermolecular interaction.
One of the three forms that matter can take - solid liquid or gas. The rate is diffusion is higher than solids and liquids. The three main states of matter are solid liquid and gas.
Usually all matters can exist in three different states- solid liquid and gaseous states. Matter can exist in one of several different states including a gas liquid or solid state. This is water with the lowest energy and temperature.
To describe the solid liquid and gas phases. Is boiling or evaporation slower. Diamond is one example.
Water oil fruit juice and many others. In everyday life we commonly come in contact with water as a solid ice as a liquid and as a gas steam. Has a definite shape and a define volume.
When water boils it will turn to vapor. The three states of matter. Liquid is a substance that flows freely having a definite volume but no permanent shape.
You just studied 17 terms. Three phases are common. The liquid state of matter becomes a solid as a result of freezing.
The gaseous state has the highest compressibility as compared to solids and liquids. Learn vocabulary terms and more with flashcards games and other study tools. Liquid vibrate move about and slide past each other.
A gas has neither a defined shape nor volume. The particular temperature for each substance at which it changes from a solid to a liquid. Liquid molecules are looser and can move about easily.
The familiar states of matter are defined not by what they are made of but mainly by. Air helium nitrogen oxygen carbon dioxide etc. We normally experience carbon dioxide CO 2 as a gas but if it were cooled down to about 78C it would become a solidThe everyday term for solid carbon dioxide is dry ice.
Gases are primarily free-flowing with little to no intermolecular force acting between them. Gases on the other hand have uniquely different properties compared to Solids and Liquids. Gas vibrate and move freely at high speeds.
When this liquid is chilled to 0C it changes to a rigid solid. Are solid liquid and gas. Liquids change into gas under the process of evaporation.
A solid that is made up of crystals in which particles are arranges in a regular repeating pattern liquid a state of matter that has no definite shape but has a definite volume. The particle model represents particles by small solid spheres. All we have to do is change the conditions of the substancetypically temperatureand we can change the phase from solid to liquid to gas and back again.
Solid are tightly packed usually in a regular pattern. For example- water liquid can exist as ice solid or water vapour gas. A solid has a defined shape and volume.
There are many different liquids such as.

Properties Of Solids Liquids Gases Compared Teachoo Science

Ks2 Science Year 4 3 States Of Matter Solids Liquids And Gases The Schools Of King Edward Vi In Birmingham
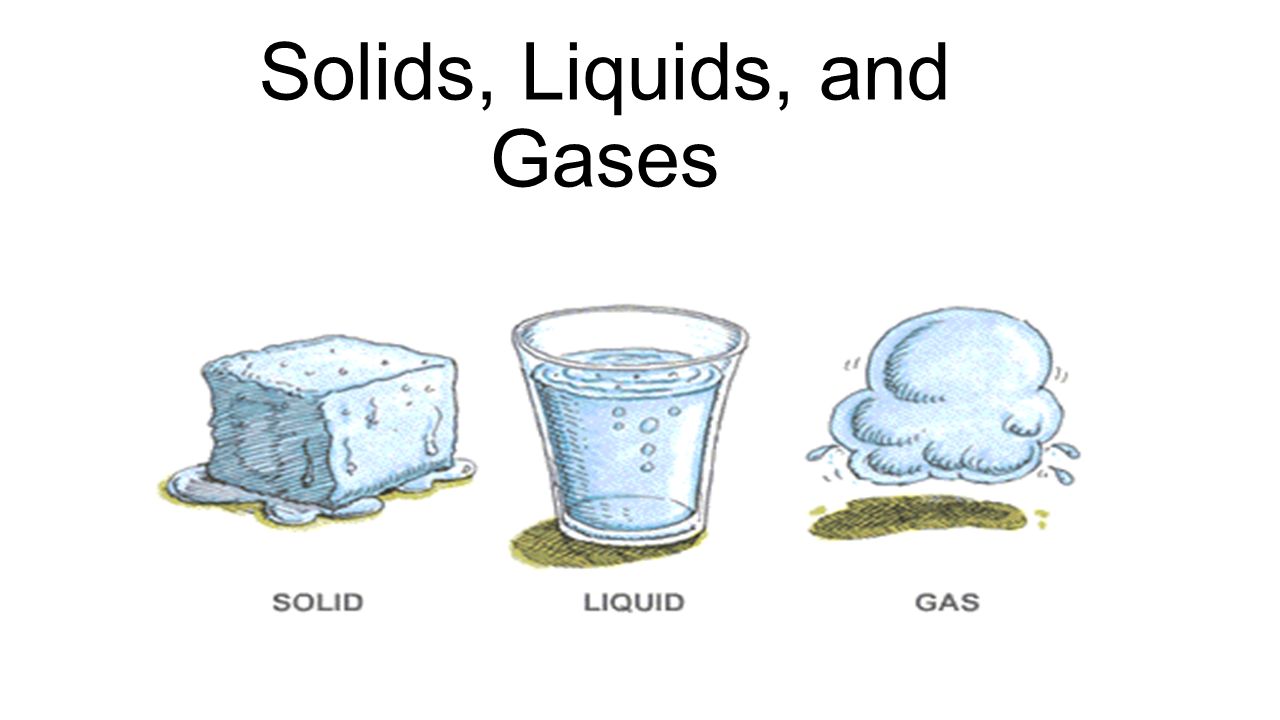
Solids Liquids And Gases Key Words Solid Crystalline Solid Amorphous Solid Liquid Fluid Viscosity Gas Ppt Download
No comments for "Describe Solids Liquids and Gases Using Scientific Terms"
Post a Comment